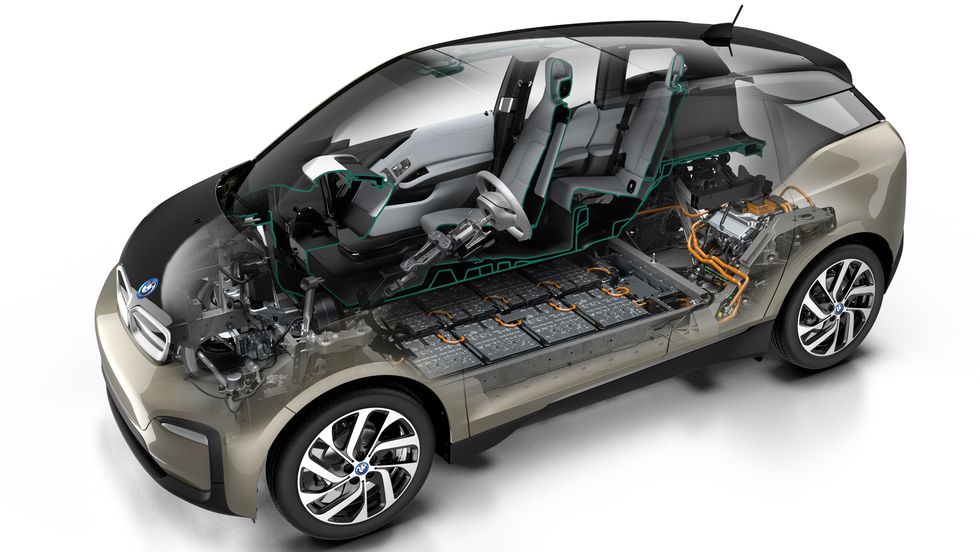
Picture a battery. It’s likely you’re envisioning a standard-format AA or AAA cell, the kind you buy to power various small electric devices, such as your television’s remote control or a smoke detector.
Now, picture the battery of an
electric vehicle. The image you’ve conjured likely looks more like a big rectangle rather than a small cylinder.
Though your mind may perceive these two types of batteries as vastly different electricity-storing devices, both the typical store-bought battery for your various electronic devices and the battery pack in an EV work on the same general principles. That said, the battery in a hybrid or electric vehicle is just a bit more complicated than those lipstick-like cells you’re used to handling.
The battery in an HEV, PHEV, or BEV (that’s hybrid-electric vehicle, plug-in hybrid-electric vehicle, and battery-electric vehicle, respectively) can be made out of a variety of materials, each of which nets different performance characteristics. The individual cells stored within these big battery packs come in many different shapes and sizes too.
How Does an EV Battery Work?
The cells within an electric vehicle’s battery pack each have an anode (the negative electrode) and a cathode (the positive electrode), both of which are separated by a plastic-like material. When the positive and negative terminals are connected (think of switching on a flashlight), ions travel between the two electrodes through a liquid electrolyte inside the cell. The electrons these electrodes give off, meanwhile, pass through the wire outside the cell.
If the battery is providing power (for instance, the bulb in the aforementioned flashlight)— an action known as discharging—then ions flow through the separator from the anode to the cathode, while electrons travel over the wire from the negative (anode) to the positive (cathode) terminal to provide power to an external load. Over time, the cell’s energy depletes as it drives whatever it’s powering.
When the cell is charged, however, electrons flow from an outside energy source in the other direction (from positive to negative) and the process reverses: electrons flow from the cathode back to the anode, increasing the cell’s energy again.
EV Battery Construction
When you think of those aforementioned AA or AAA batteries, you’re imagining a single battery cell. But the batteries in EVs aren’t a huge version of that single cell. Instead, they’re made up of hundreds, if not thousands, of individual cells, usually grouped together into modules. Up to several dozen modules can reside within a battery pack, which is the complete EV battery.
EV cells may be small cylindrical cells, like a AA or AAA cell, of various standardized dimensions. This is the approach Tesla, Rivian, Lucid, and some other automakers take, wiring together thousands of these small cells. The advantage, these companies claim, is that small cells are far cheaper to produce in volume. Still, Tesla plans to move to lower numbers of larger cylindrical cells to reduce the number of connections within their cars’ battery packs.
But EV cells come in two other formats: prismatic (rigid and rectangular) or pouch (also rectangular, but in a soft aluminum case that allows some expansion in the cell walls under extreme heat). There are few standardized prismatic- or pouch-cell dimensions, and most carmakers—General Motors and Ford, for example—spec their own in partnership with the cell manufacturer, such as China’s CATL, Japan’s Panasonic, or Korea’s LG Chem.
Types of EV Batteries
The chemistry of an electric vehicle’s battery—or the materials used in its cathode—varies among different cell types. Today, there are essentially two types of battery chemistry, both under the umbrella of lithium-ion, meaning their cathodes use lithium along with other metals.
The Two Types of Lithium-Ion Batteries
The first, most common in North America and Europe, uses a blend of either nickel, manganese, and cobalt (NMC) or nickel, manganese, cobalt, and aluminum (NMCA).
These batteries have higher energy densities (energy per weight, or energy per volume) but also a higher propensity to oxidize (catch fire) during a drastic short circuit or severe impact. Cell makers and battery engineers spend a great deal of time monitoring cells and modules, both during manufacturing and while in use over the life of the car, to limit the chance of oxidization.
The second type, far more widely used in China, is known as lithium-iron-phosphate, or LFP. (This despite the fact that Fe is the symbol for iron on the periodic table, while F is actually fluorine.) Iron-phosphate cells have considerably lower energy density, so larger batteries are needed to provide the same amount of energy (and hence driving range) as NMC-based batteries.
Offsetting that, however, is that LFP cells are less likely to oxidize if shorted. LFP cells also do not use rare and costly metals. Both iron and phosphate are used in a variety of industrial applications today, and neither is remotely considered rare or resource-limited. For those reasons, LFP cells are less expensive per kilowatt-hour.
The lower cost led Tesla (and most recently Ford) to use LFP cells in its base-model electric vehicles, saving the pricier and higher-energy chemistries for more expensive models in the lineup.
As for the other cell electrode, the anode, today most of them are made of graphite.
EV Battery Software
Unlike your basic AA or AAA cell, an EV battery requires a lot of software to keep tabs on things. You might expect a AA or AAA cell to last, at most, a couple of years. Automakers, however, warranty their EVs’ battery components, often for around a decade or as much as 150,000 miles of use.
All EV batteries lose some charge capacity over time. With limited data available, it’s difficult to dig into the specifics of these losses. In general, the loss of range after 100,000 miles might be on the order of 10 to 20 percent. In other words, an EV originally capable of delivering 300 miles of range would still net between 240 and 270 miles of range at this point in its lifecycle.
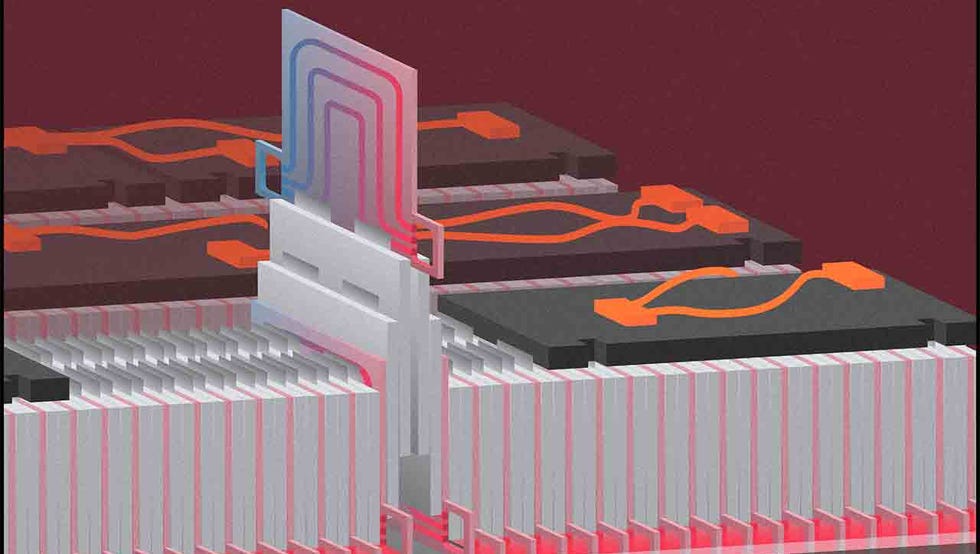
To ensure that happens, the battery modules and the pack itself have a slew of sensors to monitor the power delivered by each component—ideally, identical across all cells and modules—and the heat of the pack. A suite of software known as the battery management system (BMS) keeps tabs on this information.
Like humans, batteries are susceptible to changes in temperature, and they perform best at around 70 degrees Fahrenheit. If an EV’s battery pack shows signs of getting too hot, the BMS of most modern HEV, PHEV, and BEV batteries will circulate coolant through the pack in order to shed heat and bring the temperature closer to 70 degrees. Batteries deliver less power in extreme cold. If an EV owner preconditions their vehicle, then its control software and BMS may use grid energy (if plugged in) or perhaps some battery energy to warm up the battery. Preconditioning allows an EV battery to deliver a specific power level as soon as the driver starts off.
New Battery Technology for Electric Cars
Battery technology is always evolving. Although today’s EVs overwhelmingly use lithium-ion packs, many of tomorrow’s battery-powered cars will likely utilize packs with different chemistries. For instance, solid-state batteries that use cells with a solid electrolyte are a promising alternative that many manufacturers are investing in. In fact, Toyota plans to introduce a vehicle with a solid-state battery by the middle of the decade.
Solid-state batteries are due to offer greater energy density that ought to afford better driving range relative to a similar lithium-ion battery. This breakthrough technology still has some ways to go, though, as engineers work to bring down the material costs of producing solid-state cells. Likewise, these cells’ lifespans will need to improve dramatically in order to accommodate the thousands of full-discharge cycles of an HEV, PHEV, or BEV.
Regardless, the future for battery-powered vehicles is promising. Look for new technologies to improve the efficiency and range of electric cars, and for the costs of lithium-ion battery packs to notably fall in the coming years.
Contributing Editor
John Voelcker edited Green Car Reports for nine years, publishing more than 12,000 articles on hybrids, electric cars, and other low- and zero-emission vehicles and the energy ecosystem around them. He now covers advanced auto technologies and energy policy as a reporter and analyst. His work has appeared in print, online, and radio outlets that include Wired, Popular Science, Tech Review, IEEE Spectrum, and NPR’s “All Things Considered.”He splits his time between the Catskill Mountains and New York City, and still has hopes of one day becoming an international man of mystery.